After ten years of sharpening a sword, Dongweien, a class 1 anti-hepatitis C drug, was approved for marketing!
2020-12-22
On December 22, 2020, the State Food and Drug Administration issued an announcement that through the priority review and approval process, it approved the listing of the Class 1 anti-hepatitis C innovative drug Emitasvir Phosphate Capsule (trade name: Dongweien) declared by HEC!
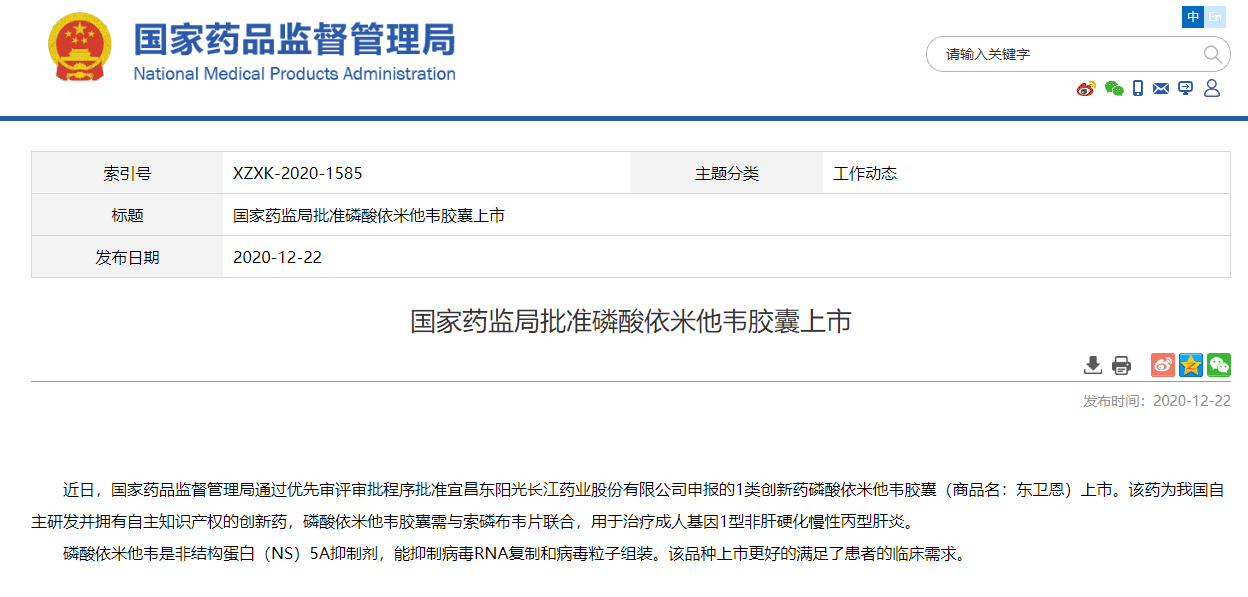
Dongweien is an innovative drug independently developed by HEC with independent intellectual property rights. It is a non-structural protein (NS) 5A inhibitor for the treatment of adult genotype 1 non-cirrhotic chronic hepatitis C. According to the data, there are still at least 10 million chronic hepatitis C patients in my country. In 2019, the WHO set the goal of eliminating hepatitis C globally by 2030. The listing of Dongweien will contribute positively to the elimination of hepatitis C in my country.
Dongweien has undergone 10 years of research and development, including clinical approval and listing approval, which takes nearly 4 years of review. The R&D team of HEC started from scratch, and the down-to-earth, conscientious and responsible work finally achieved fruitful results, and achieved the first innovative drug of HEC on the market. HEC currently has a rich product pipeline, with 20 Class 1 drugs in clinical development, focusing on three indications of anti-infection, anti-tumor and endocrine metabolism. In the future, new drug products in the pipeline will also be gradually approved for marketing.
Related news
2022-10-22

WeChat public account
2022 Copyright by HEC Group 粵ICP備19034764號(hào)-1